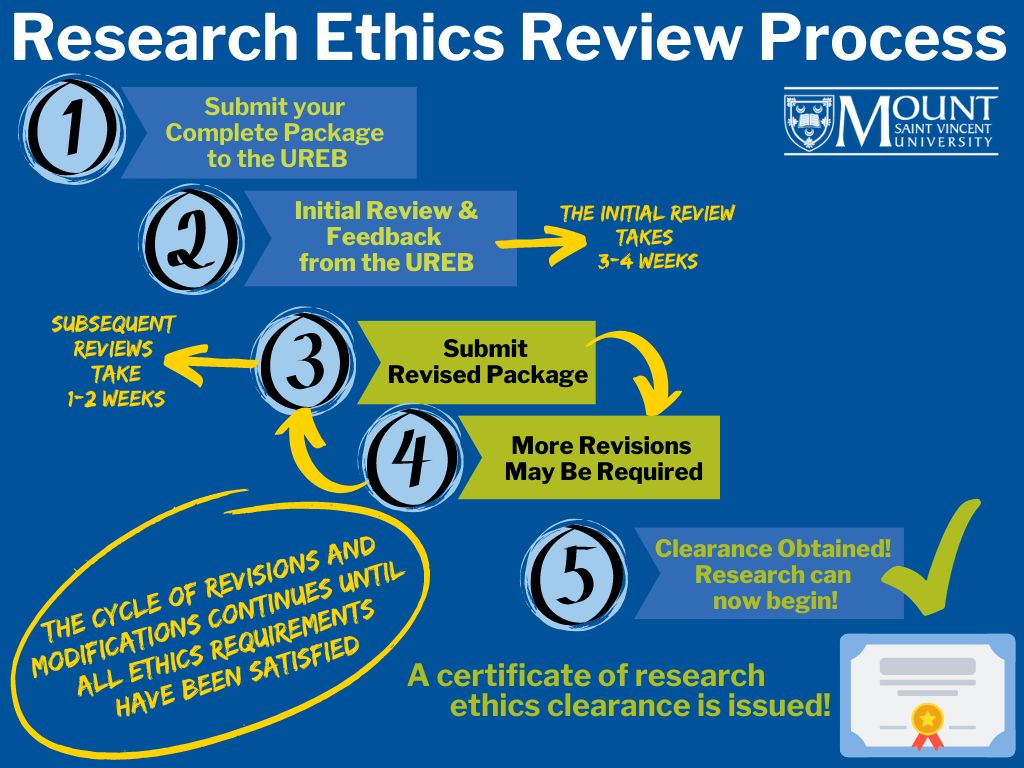
Research Ethics Review Process
> Download the Research Ethics Application Form.
> Read about the detailed process and go through the checklist – this will help ensure expedient review of the application.
> Please contact Brenda.Gagne@msvu.ca if you have any questions or concerns.
Remember: Researchers may not begin any research with human participants until ethics clearance has been issued.